Silver metal has excellent conductivity, but its properties are soft and low in strength. When using it as a conductive metal, it is often necessary to balance the strength and conductivity, but new materials may change this situation. A group of researchers managed to use defects to make silver much stronger than usual, while still maintaining considerable electrical conductivity. This discovery broke the theoretical limit of decades.
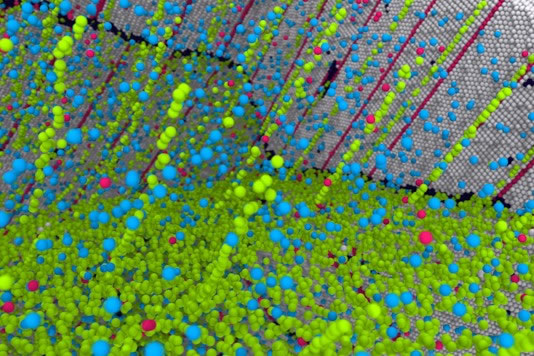
This silver crystal's atomic structure model is filled with copper impurities (green), so that their own defects are filled
Defects are an inevitable part of metals. Problems such as fragility or aging under the influence of time are common. The solution is usually to combine different metals into alloys, which helps to overcome some of these problems, but for conductive metals In general, this is usually at the expense of conductivity, and seeking the best of both worlds is the purpose of this study.
Morris Wang, co-author of the study, said: "We asked ourselves, how can we make defective materials without overcoming the softening problem while maintaining electrical conductivity?" The solution sounds very simple : They mixed trace amounts of copper into silver. The final strength is 42% stronger than the strongest silver previously, while still having electrical conductivity. But the most impressive thing about this new alloy is that it exceeds the so-called "Hall Page limit".
The Hall-Petch effect is the iconic theory of materials science for more than 70 years. This theory believes that as the metal grains become smaller, the material itself will become stronger, but there is a limitation: when the crystal When the particles become too small (a few nanometers wide), their boundaries become unstable and the material will soften again.
The researchers managed to create a material called "nanocrystalline twin metal", which broke through this limit. Since copper atoms are slightly smaller than silver atoms, they tend to fall into and fill defects in the grain boundaries. This can prevent the movement caused by the defect and eventually lead to the softening of the material. At the same time, copper does not prevent electrons from passing through silver, thereby maintaining electrical conductivity.

The research team said that this method can be applied to many other metals besides silver. The technology can eventually be used to make more efficient solar panels, lighter aircraft or safer nuclear power plants.
The study ’s first author, Frederic Sansoz, said: “This is a new class of materials, and we are just beginning to understand how they work.
Foshan City, Nanhai District Huidexing Stainless Steel Products LTD., , https://www.huidexing.net
![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)